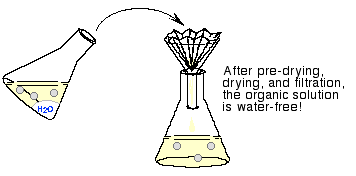
Drying
- After removing inorganic impurities by
washing, the remaining contaminant is water.
-
- Removal of water consists of two steps:
predrying
and drying.
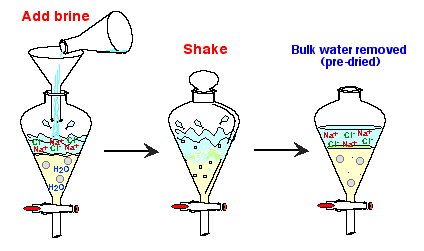
1. Predrying
- Washing a wet organic solution with saturated
aqueous NaCl solution (or "brine") removes the bulk
of water dissolved in the organic layer.
-
- Saturated NaCl solution is a very polar
medium, and water, which is also a polar compound, will move
to the polar layer, rather than staying in the nonpolar organic
medium.
-
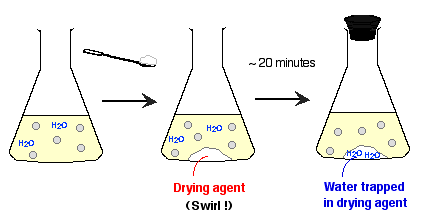
2. Drying
- To remove the trace of water remaining
after predrying, a drying agent is used.
-
- A drying agent is an anhydrous inorganic
salt, such as calcium chloride (CaCl2), magnesium
sulfate (MgSO4), and sodium sulfate (Na2SO4).
These solids are insoluble in organic solvent, and readily absorb
water to form hydrates.
-
-
- You add sufficient solid drying agent
to the organic solution, stopper the flask, then set the mixture
aside for several minutes. You can remove the hydrated drying
agent simply by gravity filtration.
-